Covalent Bonding
4.3 Covalent Bonding Learning Objectives
- Exceptions to covalent Bond
- Shape of molecules
- VSEPR theory
- Giant covalent bond
Coordinate Covalent Bond
A covalent bond is the electrostatic attraction between positively charged nuclei and shared pairs of bonding electrons.
- single bond, double bond, and triple bond
A coordinate covalent bond differs from a ‘regular’ covalent bond because both the bonding electrons come from one atom. Atoms in molecules that are electron-deficient (lacking in electrons) are able to form coordinate covalent bonds
The octet rule
The octet rule states that the most stable arrangement for an atom is to have eight electrons in its outermost energy level with the electron configuration of a noble gas. However, there are some exceptions to this rules.

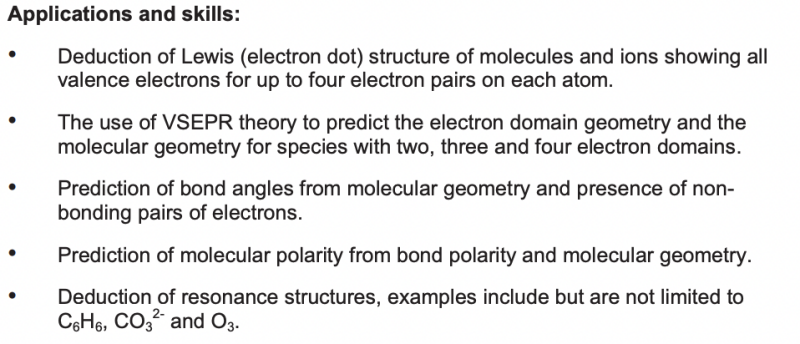
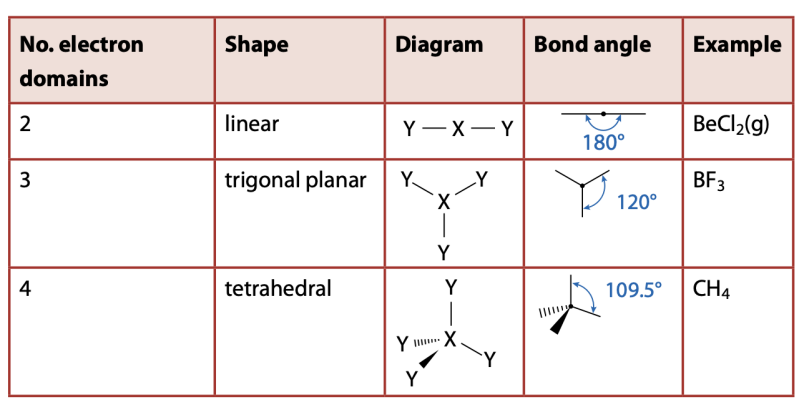
VSEPR Theory
Pairs of electrons (electron domains) in the valence (outer) shell of an atom repel each other and will therefore take up positions in space to minimize these repulsions – to be as far apart in space as possible.
Basic Shapes
Actual Shape

- Basic Shape: based on electron domains
- Actual shape: based on the number of lone pairs
Giant Covalent Bonding
Diamond
For diamond, each carbon atom is bonded to four other carbon atoms, forming a tetrahedral shape.
- Do not conduct electricity: Diamond does not conduct electricity, because all the electrons are held strongly in covalent bonds and are therefore not free to move around in the structure.
- High boiling and melting points: Diamond is hard and has a very high melting point and boiling point (about 4000 °C) because a lot of energy must be supplied to break covalent bonds (very strong) when diamond is melted/boiled.
- Not soluble: Diamond is not soluble in water or organic solvents because the forces between the atoms are too strong. The energy to break these covalent bonds would not be paid back when the C atoms were solvated.
- Hard: Diamond is hard due to the strong attraction force of giant covalent bond.
- Shiny: This is due to reflection and refraction of lights


Graphite
In graphite, each carbon atom is bonded to three other carbon atoms, forming a trigonal shape
- Soft and slippery feeling: Used in pencils and dry lubricant
- Insoluble in water and organic solvents
- Conduct electricity: because each C atom forms only three covalent bonds, so one delocalized electron (carbon has four outer shell electrons) can move within the layers.
- High melting and boiling points: because covalent bonds within the layers must be broken when it is melted/boiled.
- Solubility: Because of the strong covalent bonds between atoms, graphite is not soluble in water or non-polar solvents.

Uses of Graphite
- Writing Materials
- Lubricants: The presence of weak forces between the layers is usually given as the explanation that graphite is a good lubricant.
- Paint
- Refractory
- Nuclear Reactors
- Batteries
- Graphene Sheets
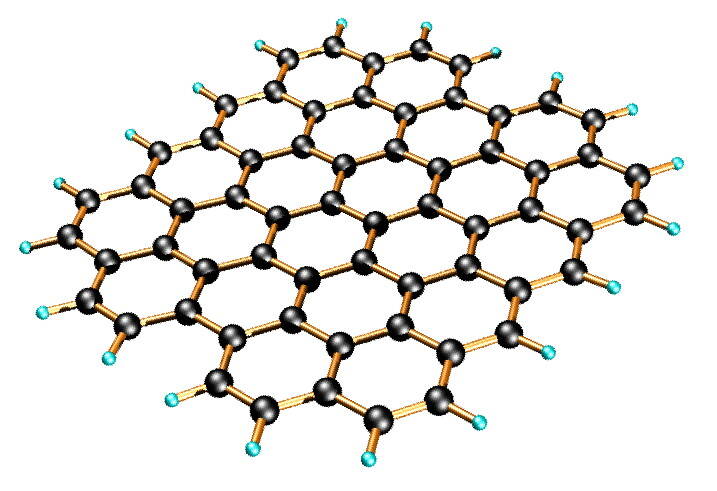
Graphene
In graphene, each carbon atom is bonded to three other carbon atoms, forming a trigonal shape
- High thermal conductivity
- High electrical conductivity
- High elasticity and flexibility
- High hardness
- High resistance
- Transparent metals
- High boiling and melting points
Application of Graphene
- Sensing: Nano and micro electromechanical systems and mechanical snesors
- Energy: energy materials, super capacitors, hydrogen storage
- Biological: biosensors, biomedicine
- Photonics: Photonic materials, lesser materials
- Materials: Oxidation, resistant packing and wiring materials
- Electronics: Data storage, transparent electrodes
C60 Fullerene (Buckminsterfullerene)
Buckminsterfullerene (C60) is a molecule that consists of 60 carbon atoms, arranged as 12 pentagons and 20 hexagons
- Do not conduct electricity
- Low melting and boiling points
- Slippery
- Insoluble in water but soluble in some organic solvents

Silicon Dioxide
In silicon dioxide, each silicon is bonded to four oxygen atoms, and each oxygen atom is bonded to two silicon atoms.
- Insoluble in acid and water, but soluble in hydrofluoric acid
- Not very reactive because of its zero polarity
- High melting and boiling points
- Does not conduct electricity

silicon dioxide (sand) exists in the building industry, e.g. for concrete production (Portland cement concrete). Silica, in the form of sand, is used as the key ingredient for the manufacture of metallic components in engineering and other applications of sand casting. The relatively high melting point of silica allows for its use in these applications.