Ionic Bonding
- Ionic bonding involves the transfer of one or more electrons from the outer shell of one atom to the outer shell of another atom. The transfer of these electrons results in the formation of positive and negative ions.
- Positive ions are known as cations and negative ions are known as anions.
- The electrostatic attraction between the positive and negative ions results in the formation of an ionic bond.
- The bonding usually occurs between a metal and a non-metal
Structure
- Ionic lattice structure: In a ionic lattice, there are strong electrostatic forces of attraction acting in all directions between the oppositely charged ions. The structure and bonding of ionic compounds explain their properties
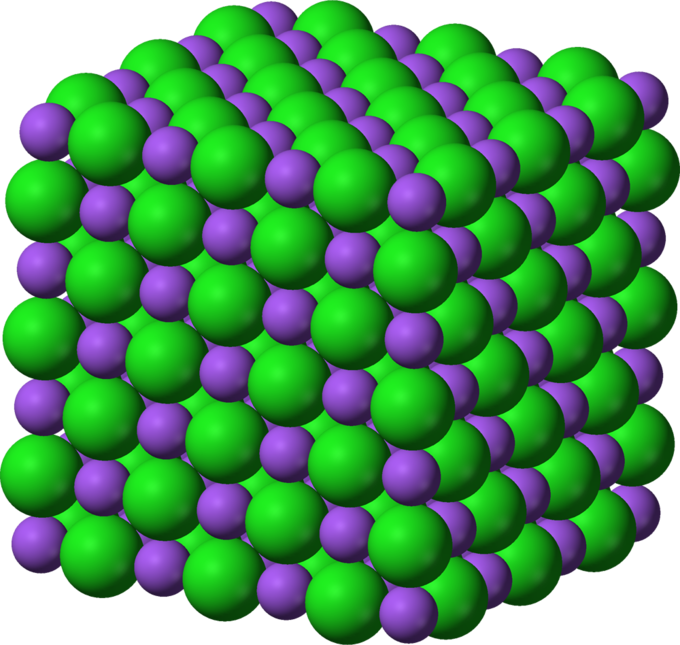
https://courses.lumenlearning.com/introchem/chapter/lattice-energy/
Properties of Ionic Bonding
- Low volatility (ability to evaporate): The volatility of ionic substances is low because the electrostatic forces between the ions are strong.
- They have high melting and boiling points: The strong attraction between the ions results in ionic compounds having relatively high melting and boiling points. This means that relatively large amounts of energy are required to break these forces of attraction between the ions.
- They're hard and brittle: Ionic compounds are usually hard due to their strong attraction force in an ionic lattice
- They conduct electricity when they are dissolved(molten) in water: Ionic compounds do not conduct electricity when solid because the ions are held in fixed positions in the lattice structure. When an ionic compound is melted (molten) or dissolved, the ions are free to move and carry an electric current
- Solubility in water: Ionic substances are often soluble in water. Water is a polar solvent, and energy is released when the ions are hydrated by being surrounded (ion-dipole attractions) by water molecules. This energy pays back the energy required to break apart the ionic lattice.
- Not soluble in non-polar solvents: This is because a great deal of energy is required to break apart the ionic lattice and this is not paid back by the energy released when the non-polar solvent forms interactions with the ions.
