Equilibrium
Notes
Important Terms
- In dynamic equilibrium, macroscopic properties are constant (concentrations of all reactants and products remains constant) and the rate of the forward reaction is equal to the rate of the reverse reaction.
- Note: Constant concentration does not mean equal concentration

Figure 1: Graph showing how the rates of the forward and reverse reactions change as a reversible reaction comes to equilibrium.
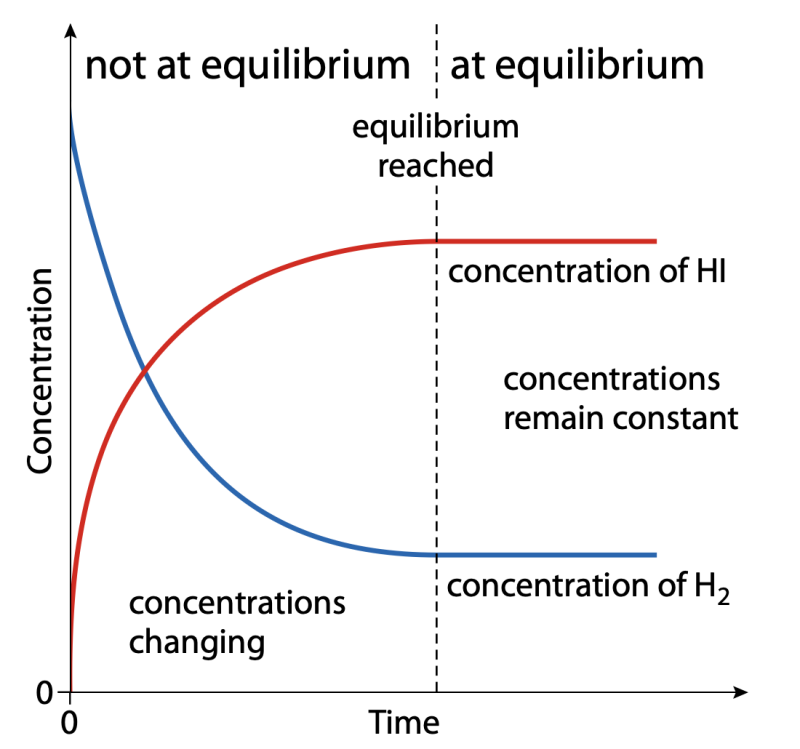
Figure 2: Graph showing how the concentrations of hydrogen and hydrogen iodide change with time.
Characteristics of Equilibrium
- Macroscopic properties are constant at equilibrium – at equilibrium the concentrations of all reactants and products remain constant.
- At equilibrium the rate of the forward reaction is equal to the rate of the reverse reaction.
- Equilibrium can be attained only in a closed system.
- All species in the chemical equation are present in the equilibrium reaction mixture
- Equilibrium can be attained from either direction
Position of Equilibrium (Le Chatelier’s principle)
- The ‘position of equilibrium’ refers to the relative amounts of reactants and products present at equilibrium.
- Equilibrium does not imply 50% reactants and 50% products.
- If a system at equilibrium is subjected to a change, the position of equilibrium will shift in order to minimize the effect of the change.
Temperature
- As temperature increases, the position of equilibrium shifts in the endothermic direction to take in heat to minimize the effect.
-
Heat reaction mixture: position of equilibrium is shifted in the endothermic direction.
-
Cool reaction mixture: position of equilibrium is shifted in the exothermic direction.

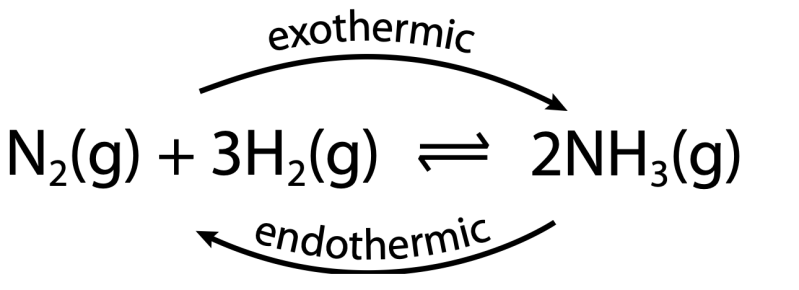
Pressure
- If a reaction involves a change in the number of gas molecules, an increase in pressure results in the position of equilibrium shifting in the direction that gives a decrease in the number of gas molecules.
Concentration
- If the concentration of one of the species in an equilibrium mixture is increased, the position of equilibrium shifts to the opposite side to reduce the concentration of this species.