Measuring Energy Changes
Some Important Definitions
- Heat is a form of energy that flows from something at a higher temperature to something at a lower temperature.
- Temperature is a measure of the average kinetic energy of particles.
- Enthalpy is the sum of the system's internal energy and the product of its pressure and volume. In simplified terms, it is the energy stored in chemical bonds
Exothermic and Endothermic Reaction
In an exothermic reaction, heat energy is transferred from a system (chemical reaction) to the surroundings: the surroundings get cooler.
In an endothermic reaction, a system (chemical reaction) takes in heat energy from the surroundings: the surroundings get cooler.

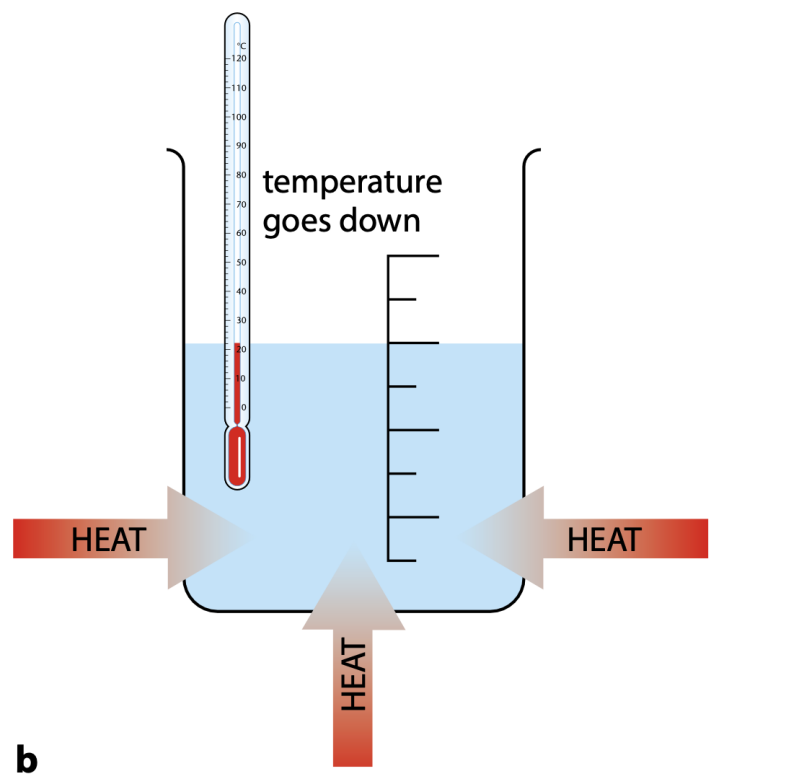
Properties of exothermic reaction are
- ∆H for an exothermic reaction is negative, meaning that products have less energy than reactants, so it is chemically stable.
- Frequent exothermic reactions include: bond formation, combustion, neutralization
Properties of endothermic reaction are
- ∆H for an exothermic reaction is positive, meaning that products have more energy than reactants, so it is chemically less stable.
- Frequent endothermic reactions include: bond breaking, vaporization (liquid to gas), melting, (solid to liquid)

- The above figure shows energy level diagram of an exothermic reaction, in which products have less energy than reactants.

- The above figure shows energy level diagram of an endothermic reaction, in which products have more energy than reactants
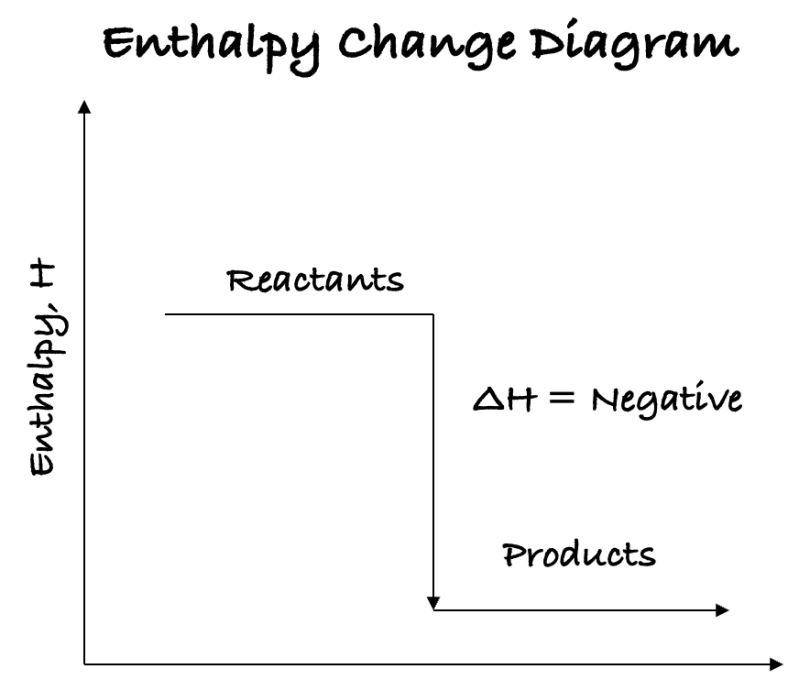

- The above figure shows enthalpy level of an exothermic reaction. Exothermic reactions have a negative enthalpy because products have less energy than reactants.
- The above figure shows enthalpy level of an endothermic reaction. Endothermic reactions have a negative enthalpy because products have more energy than reactants.
- Standard enthalpy change of reaction (∆H) is the enthalpy change when molar amounts of reactants react together under standard conditions to give products.
-
Standard enthalpy change of combustion (∆H) is the enthalpy change when one mole of a substance is completely burnt in oxygen under standard conditions.