Atomic, Nuclear, and Particle Physics
- Discrete energy and discrete energy levels
- Transitions between energy levels
- Radioactive decay
- Fundamental forces and their properties
- Alpha particles, beta particles and gamma rays
- Half-life
- Absorption characteristics of decay particles
- Isotopes
- Background radiation
- Describe and explain gas spectra in terms of energy levels
-
Solve problems with atomic transitions.
-
Describe the fundamental forces between particles.
-
Describe radioactive decay, including background radiation, and work with radioactive decay equations.
-
Describe the properties of alpha, beta and gamma particles.
-
Understand isotopes.
Atoms and Nuclear Structure
An atom is made up of proton, electron, and electrons. The atomic number Z is equal to its proton number, electron number is equal to proton number, and mass number A is equal to the number of neutron (N) plus the number of proton(N = A-Z).

Atoms with the same number of proton by different number of beutron is knows as an isotope. For example, carbon-12 and carbon 13. Elements in a periodic table are arranged by atomic (proton) number, so changing an atomic number changes the particle.
Discrete energy and Discrete Energy Levels
Electrons in an atom are arranged in energy levels (shells) around the nucleus. The first shell is known as the K shell, closest to the nucleus. The shells increase in energy as they get further from the nucleus. The maximum number of electrons in each shell is given by 2n ^2 .


Figure 2: Demonstration of energy level in an atom.
Emission and Absorption Spectrum
- Light is electromagnetic radiation that human eyes can detect. Light with different colors has a different wavelength and frequencies, shown by an electromagnetic spectrum.
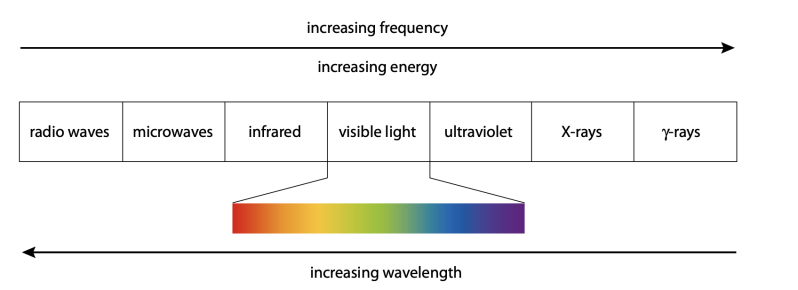
- Each element has their unique wavelength, and the set of possible wavelengths that can be emitted is called an emission spectrum. As lines are not continuous in the spectrum, it can be concluded that atoms have discrete energy levels.

Figure 5: Different spectrums.
- Light can be seen as a form of energy given off by photons.
- Absorption: Absorbing photons: an electron absorbs energy and jump from a lower energy to a higher energy level.
- Emission: Emitting photons, an electron releases energy, jumping from a higher energy level to a lower energy level.
- Energy: The energy released or absorbed is calculated by E=hf, where h is a Planck's constant, and f is frequency. From the equation, we can conclude that a longer wavelength means a lower energy, and vice versa. A higher frequency means a higher energy.


Figure 6: Electron Transition
Figure 7: Three key equations
Energy Level Diagram
- The transition from a higher to a lower or from a lower to a higher energy is often represented by an energy diagram.
- Convergence: line spaces decreases as the number of n increases, meaning that energy converges at a higher frequency
- Arrow length and energy: A longer arrow represents a larger amount of energy, and a shorter arrow represents a lower amount of energy
- Arrow direction: Arrows pointing downward means photons are emitted and energy is released. Arrows pointing upward means photons are absorbed and energy is absorbed.
- Wavelength: The total number of possible transitions represents the total number of possible wavelengths.

Figure 9: An energy diagram and explanation. Emission Spectrum Quiz (Paper 1)
Radioactive Decay and Half Life
- The emission of particles and energy from a nucleus is called radioactivity. The emissions are called alpha particles, beta particles and gamma rays. A alpha particle is identical to a helium atom (2 protons), ,and a beta particle is an electron.

Figure 8: Fundamental particles
Alpha Decay
- An Alpha particle is identical with a helium atom that has a proton number of 2 and an atomic mass of 4.
- An alpha particle is emitted from the nucleus and the decaying nucleus turns into a different nucleus
- Proton number (Z) decreases by two, and mass number decreases by four.
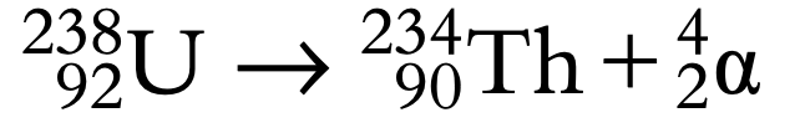
Figure 10: An example of Alpha decay, uranium decaying into thorium:
Beta Particle and Beta Decay
- In beta minus decay, a neutron in the decaying nucleus turns into a proton, emitting an electron and an anti-neutrino.
- Proton number increases by one, and mass number remains unchanged

Figure 11: An example of beta minus decay, nucleus of thorium decaying into a nucleus of protactinium
Beta Particle and Beta Decay
- he nucleus emits its anti-particle, the positron, forming a new particle, proton (the 2nd particle, a positively charged particle), and a neutrino.
- The proton number of the left-side particle decreases by one and mass number remains unchanged, forming the new particle

Figure 12: An example of beta plus decay
Gamma Decay
- In gamma decay a nucleus emits a gamma ray (a photon of high-frequency electromagnetic radiation).
- Proton number and mass number remain unchanged after the decay.

Figure 13: An example of gamma decay