Modeling a Gas
Learning Objectives
- Use the concept of pressure
- Solve problems using the equation of state of an. idea has
- Understanding the assumptions behind the kinetic model of an ideal gas
- Solving problems using moles, molar mass, and the Avogadro constant
- Describe differences ideal and real gases
- The Avogadro constant
- Ideal Gases
- The Pressure-Volume Law
- The Pressure-Temperature Law
- The Boltzmann Equation
The Avogadro Constant
- Avogadro's number is the number of units in one mole of any substance , equal to 6.02214076 × 1023
-
A mole is a unit of measurement, equal to 6.02214076 × 1023. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12
-
Molar mass is the mass of one mole of a substance
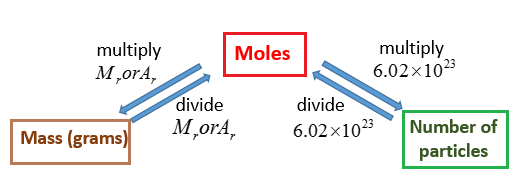
https://www.onlinemathlearning.com/grams-moles.html
Pressure


Ideal Gases
Ideal gases are theoretical model to gases, and they are assumed to obey the followings
- The particles are point particles, each has a negligible volume
- The molecules obey the law of motion
- The are no forces between molecules unless when they collide (molecules do not attract or repel each other)
- The duration of collision is negligible compared with the time between collisions
- The collisions with molecules and other contain walls are elastic
- Molecules have a range of speed and move randomly
An ideal gas can be approximated by an ideal gas when the density is low or when at a high temperature and low pressure. This is because molecules are distant away from each other when density is low, so molecules will exert a weaker force on each other.
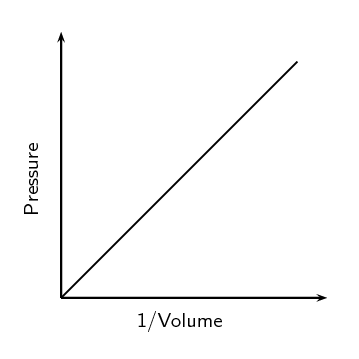
The Pressure - Volume Law (Boyle's Law)
- At constant temperature and with a fixed quantity of gas, pressure is inversely proportional to volume and pressure times volume is equal to a constant.



Charles' law
-
The volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.


Figure 4: Temperature in Celsius

Figure 5: Temperature in Kelvin
The Pressure-Temperature Law
- For a given mass and constant volume of an ideal gas, the pressure exerted on the sides of its container is directly proportional to its absolute temperature.


Ideal Gas Equation
- The state of gas can be determined when we know the volume of pressure P, volume V temperature T, and the number of moles present n. The equation related all quantities is known as the equation of state, as shown below.

Gas Laws Summary
